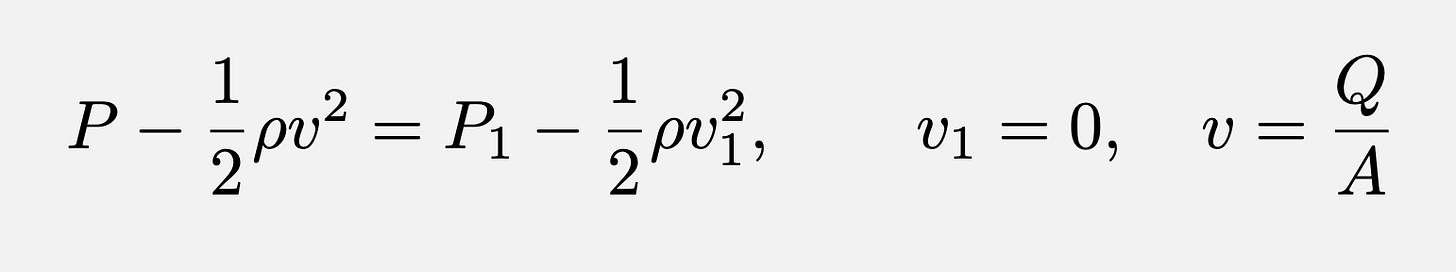Summary
This is the fourth Substack post of a series of posts describing vaping aerosols, their properties, their optimal regime of operation and comparisons with tobacco smoke and other aerosols. Understanding how vape aerosols form, operate and can be tested provides the knowledge to understand their pleasurable usage, their toxicity profile and relative safety with respect to tobacco smoke and other aerosols and pollutants. Without being “experts” this knowledge reassures our confidence on the role of vapes in harm reduction and serves us to counter ignorant and malicious disinformation.
This knowledge (which i try to present accessibly) reassures our confidence on the role of vapes in harm reduction and serves us to counter ignorant and malicious disinformation.
Previous posts: Post 1, Post 2, Post 3
Post 4:
I described in Post 3 the thermal processes taking place under the conditions of an Overheating Regime whose end point is a “dry puff”.
In this Post I explain how the parameters for operating vape devices are set up for laboratory testing and why the current standard needs to be upgraded for this testing to achieve valuable technical information to establish quality control standards and evaluate their safety profile.
Basics of laboratory testing
Since instruments cannot be placed in human mouths and throats, vape aerosols to be analyzed in the laboratory must be generated by “vaping machines” that simulate the inhalation though a pump mechanism . These vaping machines evolved from machines traditionally used by the tobacco industry to test cigarettes. They can be programmed to activate any vape device, to operate it at different puffing times, inter-puff lapses, supplied power and airflow rates.
Laboratory testing is very useful to manufacturers, vape shops, health professionals, regulators and consumers, providing valuable technical information that serves to establish quality control standards. Given the wide variability of devices, coils, e-liquid mixtures, nicotine levels and flavor chemicals, the results of these tests are also useful for comparison between devices and components. The chemical analysis of the generated aerosol provides valuable information to assess their safety profiles. All these issues are crucial for the regulation of the devices and liquids.
For quality standards and inter-product comparison laboratory testing must be standardized, with tests that conduct blocks of regimented puffs with same parameters, with a sufficient number of puffs per block to statistically validate the results. Obviously, regimented puffing does not reproduce user patterns, but if the “topography” (puffing parameters) is appropriate, the laboratory protocols can be reasonable proxies of consumer usage to assess risks and potential harms from solvents (PG and VG), nicotine, toxic byproducts and flavor chemicals. It is in this issue where the conditions for the Optimal Regime are very relevant.
Early experiments & the “formaldeyde scare”
Earlier studies on vape aerosols (before 2014) tested first generation cigalikes, low powered closed devices shaped as metallic cigarettes. The devices had several faults in their design, solders, wires and plastic elements were in contact with the e-liquid, nicotine delivery was poor and flavorings were not appealing (likely because of leaking of internal components into the liquid) .
Even considering these drawbacks, their experimental outcomes persistently revealed concentrations of aldehyde byproducts to be well below their levels in tobacco smoke. Although some of these studies are still cited, their outcomes only apply to devices that are now obsolete and are thus no longer relevant.
Between 2014 and 2016 second generation new devices emerged. They were open refillable devices resembling fountain pens. The wetting of the wick was improved and some devices allowed users to modify voltage and use ventilation slits to modify the airflow.
In 2014-2016 several emission studies reported alarmingly high levels of the three main aldehyde byproducts (see Post 2, Post 3). In particular, a “formaldehyde scandal” emerged after a study by chemists from the University of Portland reported formaldehyde precursors (which the authors described as “hidden formaldehyde”) at extremely high levels, well above the levels in tobacco smoke.
Some of the studies reporting high content of aldehydes used the so-called “top coil” devices, with the wick and coil on the upper part of the atomizer. As shown in Figure 1 below, This design makes it harder for the e-liquid to wet the wick (against gravity), with high likelihood of the coil heating a semi-dry wick. Also, the conduct to evacuate (forced convection) the vapor is too short for its effective cooling and condensation. This design was prone to overheating and high byproduct generation, so it was abandoned.
Early first generation vape devices operated in narrow power ranges and had no user control of these ranges. Nevertheless, human users would notice a progressive taste deterioration when of e-liquid levels begin to drop and a full burning taste on its depletion, identifying this phenomenon as a “dry puff” and immediately discontinue usage. Evidently, vaping machines kept puffing under these conditions.
The Portland University study and other similar studies were replicated by Konstantinos Farsalinos and coworkers, showing that such high levels of aldehydes only occurred under machine generated aerosols that were repellent to users and under a “dry puff” as end product of the Overheating Regime I described in Post 3.
In the devices tested in 2014-2016 the dry puff was regarded as a sudden instantaneous event as e-liquid depletes, when it is just the endpoint of an Overheating Regime (as described in Post 3). Although this regime is very rapid in low powered devices, it can also be tested with the functioning curves described in Post 3. The confusion occurs because users of these low powered devices had no control over supplied power, thus e-liquid depletion happened very fast after the initial perception of a foul taste.
In 2018 Farsalinos and Guilman published a landmark review of 32 emission studies published between 2013 and 2017 that focused on the detection of carbonyls (organic chemicals with double carbon-oxygen bounds that include aldehydes) in vape aerosols. This review showed a literature with an erratic wide diversity of experimental standards (puffing protocols and analytic techniques), while most reviewed authors were unaware of the possibility that their vaping machines could be producing unrealistic aerosols under dry puff conditions. Unfortunately, this problem and the lack of a universal testing standard persists up to the present date.
Besides carbonyls, the presence of byproducts of flavor chemicals and metal elements in the aerosol can be concerning. We deal with these byproducts in future posts.
The CORESTA standard
The only universally recognized standard on laboratory tasting of vape aerosols and liquids was introduced by the Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA), an association whose members are companies, institutes, laboratories, organizations staging Research & Development activities on tobacco or its derived products (including “e-vapor products”). Its purpose is to promote and facilitate international cooperation and best practices in scientific research.
Part of the CORESTA strategy has been initiating specific activities (Workstreams) undertaken by working groups in a collaborative manner, producing technical Reports, databases, guides, peer reviewed articles and CORESTA Recommended Methods (CRMs). The EVAP (“e-vapor”) sub-group founded in 2016 deals with vapes (devices, aerosols, e-liquids), generating 5 guides, 5 CRMs and 13 technical reference reports. Among its technical documents dealing with a variety of issues (including chemical analysis), the most relevant CRM for guiding aerosol generation through vaping machines is the CRM No. 81 paired with the ISO 20768 standard, whose tasks are “Routine Analytical Machine for E-Cigarette Aerosol Generation and Collection - Definitions and Standard Conditions”.
The CRM No. 81 was conceived as an adaptation to early vape devices of the CRM No. 22 (ISO 3300) standard for laboratory testing of tobacco smoke through smoking machines. Since users were overwhelmingly smokers or recent ex-smokers, it was understandable to recommend puffing parameters not much different from those of smoking:
puff duration 3 s
inter-puff lapse 30 s
puff volume 55 mL
airflow rate 1.1 L/min
These puffing parameters are consistent with the “mouth to lung” vaping style with retention at the oropharyngeal cavity before lung inhalation, similar to the puffing ritual used by many smokers.
Evolution of the vape market
Laboratory testing of vape devices is useful in as much as it reflects tendencies and habits of consumer usage. Unfortunately, testing standards (including CORESTA) have not kept up with the pace of evolution of the market.
From 2015 onward a wider variety of devices emerged. While smokers continued switching to vaping and required a tobacco cigarette as a useful reference, millions of vapers had already switched away from smoking and did not require vapes that shared characteristics with cigarettes. These vapers not only welcomed a wider variety of liquids and nicotine levels, but also more flexible (even modular) designs of the devices, which required different puffing habits, as well as physical changes in the device characteristics: batteries, resistances, supplied power and airflow.
The exhalation of larger thicker aerosol clouds requires the generation of a larger amount of e-liquid vapor, which involves higher supplied power to the coil. This extra vapor requires the user to do a much deeper inhalation (applied airflow) to efficiently evacuate, cool and condense this large amount of e-liquid vapor. All this required new devices whose design had to modify the physical parameters of the devices. As shown in Figures 2, 3 and 4 of Post 2, pleasurable vaping with thermodynamic efficiency requires to match high power (above 40 W) with lower resistance (below 1 Ohm), low nicotine concentration and wide mouthpieces that reduce air resistance to allow for a deep “direct to lung” (large airflow) that bypasses the throat retention of the “mouth to lung” style.
Modular third generation “mods” became popular between 2016 and 2019, but only a minority niche of dedicated veteran users generated huge clouds through very deep inhalation from devices (called “sub-ohm”) with very high power (40-200 W), very low resistance (below 0.3 Ohm) and typically e-liquids with higher VG and low nicotine levels. However, most vapers adopting modular tank models simply wanted just a bit more of a cloud and extra flexibility (power and airflow control) in comparison with claromizers and cartomizers. Nevertheless, the passage from second to third generation tank devices did involve a widening of the ranges of power (10-20 W) and inhalation deeper than that of early devices.
However, from 2019 onward, consumer tendencies strongly shifted towards lower powered tank devices (which remained popular). Also, a new fourth generation of devices called “pods” emerged that combined the compactness of early cigalikes and cartomizers with more advanced design and even user control. Some pods were refillable and some used replaceable cartridges (Juul), some operated with nicotine salts at high concentrations, some at very low power, but some were able to operate at mid range power levels (10-40 W). Since 2021 a new type of devices emerged: the single use disposables, which roughly follow the pod design, but are not refillable nor use cartridges.
The design of the new devices involves a wide variety of modifications of functional parameters (power, coil resistance, airflow, nicotine levels, air resistance), as well as modifications of users’ rituals that define “vaping styles”, including intermediate puffing styles between the extreme of very tight “mouth to lung” (narrow mouthpieces and low power/airflow) and the outgoing huge clouds of “direct to lung” (wide mouthpieces and high power/airflow).
Is the CORESTA standard still appropriate?
The CORESTA standard (CORESTA Recommended Method No. 81, ISO 20768) places no limitations on supplied power and coil resistance. However, its appropriate applicability to the wide variety of currently available devices (as described above) is severely restricted by its fixed airflow rate of 1.1 L/min.
The airflow rate of the aerosol in the mouthpieces is an important factor that characterizes vape devices. Inhalation expands the lungs and decreases the pressure inside them with respect to atmospheric pressure. This negative pressure difference is the “pressure drop” that drags the aerosol out of the mouthpiece (assuming it to be a cylindrical conduit). Assuming constant aerosol density and laminar flow without viscosity, energy conservation inside the mouthpiece (the Bernoulli equation) relates the pressure inside the mouthpiece P and the square of the aerosol velocity at initial time (before inhalation “1”) and at final time (after inhalation “2”) through the formula
where P1 is the atmospheric pressure and v1 = 0 because the aerosol does not move when the pressure drop is zero (pressure inside the mouthpiece is atmospheric pressure). Other symbols are Q is the airflow rate (volume/time =L/min) and A is the conduit (mouthpiece) cross section area. Air resistance Ra measures the proportionality between the pressure drop P-P1 and airflow rate Q from the formula written above
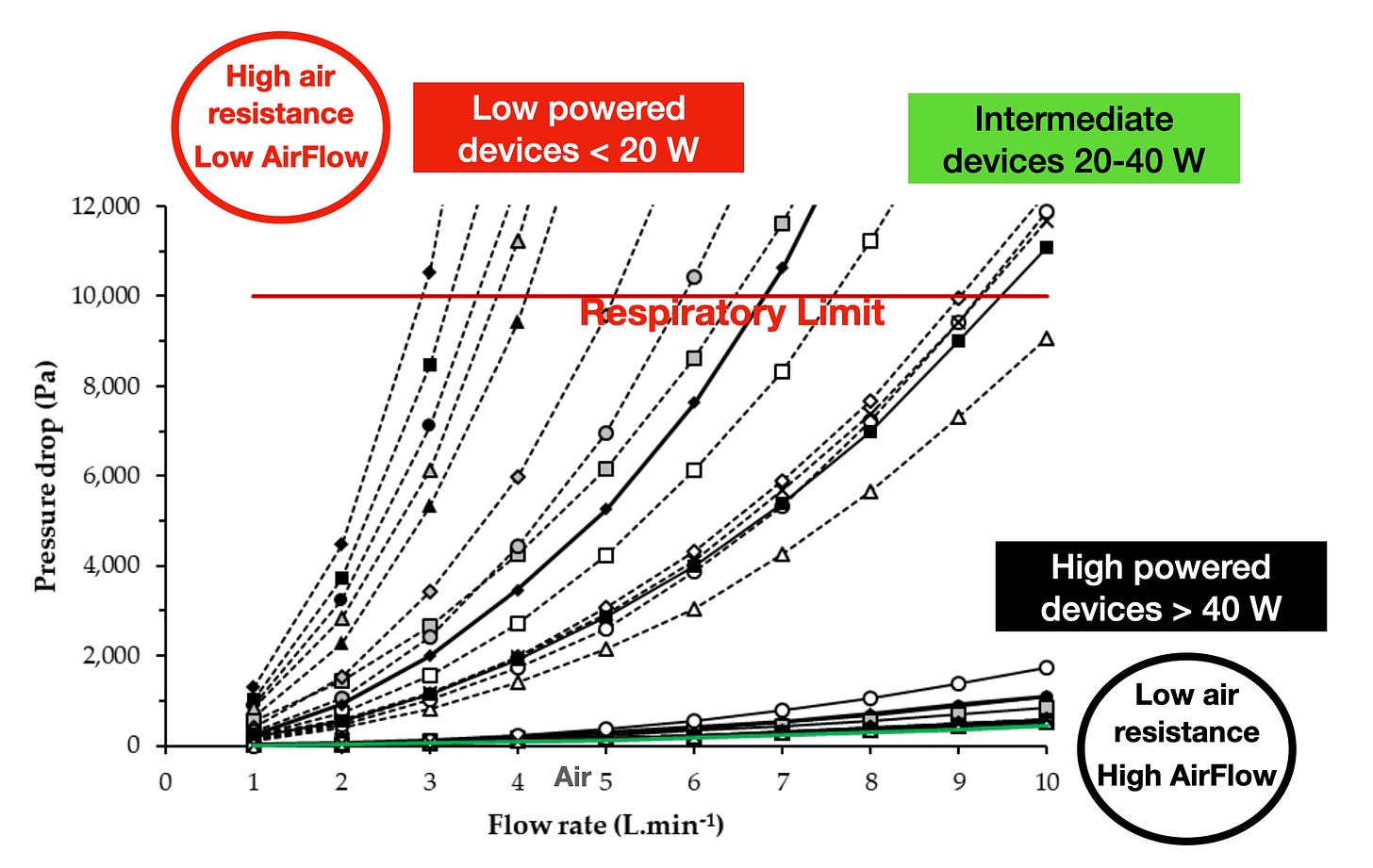
This formula (an approximation to the Darcy-Weisbach equation) shows how a large air resistance Ra corresponds to a narrow mouthpiece (small A) and requires a large pressure drop. Likewise, small air resistance Ra corresponds to a wide mouthpiece (large A) and requires much less pressure drop. This relation was plotted experimentally by Soulet et al for many individual devices
where the respiratory limit 10,000 ppa (102 cmH20) is the maximal possible pressure drop in males (it is 6500 in women)(reference). These measurements show to what degree the CORESTA rate of 1.1 L/min can be restrictive:
All low powered devices (Juul, Blu, curves in the left) can be puffed with moderate effort (20% of maximal pressure drop) at airflow rates up to 2 L/min.
Devices in the intermediate power range 10-40 W can be puffed even with moderate effort at airflow rates between 3-6 L/min.
High powered devices (curves below the diagram) can be puffed at high airflow rates close to 10 L/min with minimal effort.
The physical relation between air resistance and airflow rate shows that (in theory) any device can be puffed with any airflow rate, but respiratory mechanics show that only for low powered devices the CORESTA airflow of 1.1 L/min is consistent (natural and comfortable) and thus involves realistic testing conditions. For many intermediate power devices and for all high powered sub-ohm devices this airflow rate is too low. In particular, for lab testing of sub-ohm devices the CORESTA airflow 1.1 L/min involves completely unrealistic conditions that bear no relation with consumer usage for ‘direct to lung’ vaping (and are at odds with the physics of respiratory mechanics).
Finally, to illustrate how the functional curves of the Optimal Regime can be useful in lab testing, consider the Cubis (Joyetech), an intermediate device with standard coil resistance of 1 Ohm. This device can be used with low airflow (‘mouth to lung’ puffing style) or with high airflow (‘mouth to lung’ puffing style). However, for laboratory testing that avoids overheating (Optimal Regime) the power ranges should be known beforehand. The functional curves for this device were obtained by Soulet et al
For the CORESTA airflow (low regime 1.1 L/min) the device should be tested in the range 7-28 W, while for the airflow rate 10 L/min (intense) the range is 9-40 W. The manufacturer recommendation is 10-25, which roughly corresponds to the CORESTA airflow, though as show in Figure 2, it can be puffed with modest effort at 5-6 L/min, avoiding overheating at larger power levels. This information is hidden if testing is restricted to a fixed CORESTA 1.1 L/min airflow rate.
Conclusions and further posts.
Testing vape devices in the laboratory is important to assess their quality control, regulation and infer their safety profiles. Puffing protocols should convey as best as possible consumer usage. Although there is only one accepted testing standard based on CORESTA Recommended Method (CRM) No. 81, most emission studies still use arbitrary puffing parameters, typically defined as small ad hoc variations around the CRM No. 81 parameters. The CRM No. 81 parameters are less appropriate for devices at intermediate power ranges (15-40 W) and are completely inappropriate for high powered sub-ohm devices. Unfortunately, there are many emission studies testing sub-ohm devices at high powers with the airflow rate of the CRM No. 81 (or small variations). As we show in the next post, these studies were conducted under clear overheating conditions, hence their reported high toxicity content is completely unreliable.