Post # 7. In vitro and in vivo systems exposed to aerosol generated by high powered vape devices
A review of 40 studies
Summary of previous posts:
Post 1. Basics: what is an aerosol? Vapes and kettles. Byproducts of the heating process. Post 2. Physical processes in vaping. Essentials of laboratory testing. Optimal Regime. Post 3. Overheating: exponential production of toxic byproducts and the “dry puff”. Post 4. Laboratory testing. The CORESTA standard and the evolution of the vape market. Post 5 Metals in vapee aerosols. Emission studies detecting metals. Our review of metal studies. Post 6 Organic byproducts, comparison with tobacco smoke, our review of the literature.
Background for Post 7
Despite their limitations, studies on exposure of in vitro and in vivo systems to vape aerosols are very important tools to understand the safety profile of vaping. Together with the application of appropriate biological protocols, experimental procedures and techniques, the appropriate experimental procedures to generate these aerosols is a crucial component to determine the quality of these studies.
The outcomes of the exposure of in vitro and in vivo systems to aerosols generated under unrealistic or overheated conditions are not useful, even if excellent and efficient procedures and techniques are applied to assess biological effects.
As explained in previous posts (Post 2, Post 3, Post 4), the relation between supplied power and airflow rate in laboratory experiments is crucial to generate vape aerosols under an the Optimal Regime (linear relation between mass of e-liquid vaporized vs supplied power). As discussed in Post 4, Post 5 and Post 6, there is full certainty of overheated aerosols from the combination of high power (> 40 W), low coil resistance (< 1 Ohms) and CORESTA and similar airflow rates (1-2 L/min).
Our literature review
In this post I present and summarize the results of a literature review written by Sebastien Soulet and myself that is currently under peer review ( https://www.qeios.com/read/9XT2GU). Our literature search found 40 studies exposing in vitro and in vivo systems to vape aerosols that were generating through the same experimental instrument, the InExpose manufactured by SCIREQ (Scireq®, Montreal, QC, Canada).
Initially we tried finding studies using the InExpose through a PRISMA seach, but this search was unsuccessful because important key words (InExpose, SCIREQ, third generation, Evic, etc) do not appear in the articles titles. We also decided to focus on studies using a coil with 0.15 or 0.2 Ohms. In the end we relied on Google Scholar which can look for specific words inside the articles, but we still needed to filter the search by hand because of Google Scholar limitations.
Of the 40 preclinic studies we found in the search only 3 studies (denoted as “Certain”) provided full information on the experimental procedures of aerosol generation (coil resistance, supplied power or voltage, airflow rate, puff duration and frequency, or coil temperature). Other 11 studies (“Almost certain” and “Suspicious”) provided sufficient information to be able to infer the missing parameters. The information of the remaining 26 studies is so scant that any assessment on them would be speculative. The 14 studies in which we found reliable (direct or indirect) information on their aerosol generation procedures are listed in the table below.
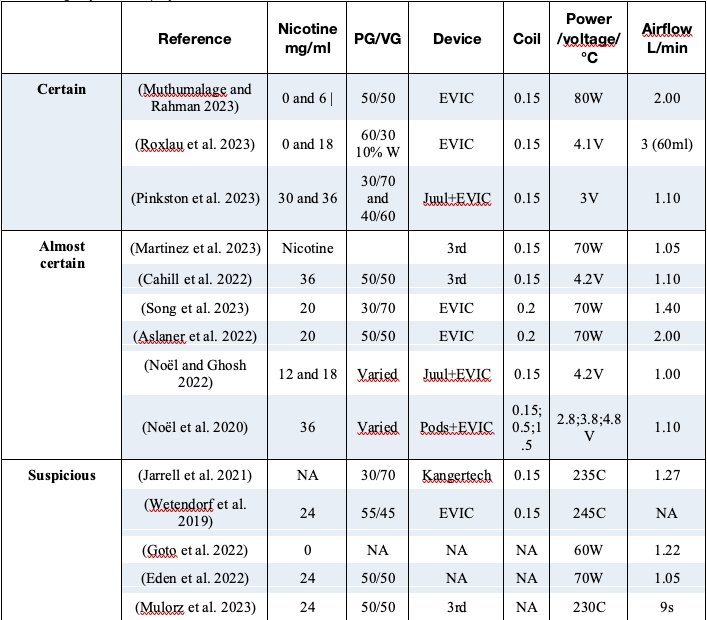
The list of the 40 studies can be found numbers in the preprint in Qeios. Notice that 9 of these 14 studies used e-liquids with nicotine concentration above 20 mg/mL, which consumers do not use in high powered devices.
The InExpose of SCIREQ
The InExpose is a computerized system manufactured by SCIREQ (Scireq®, Montreal, QC, Canada) for the purpose of conducting experiments to evaluate the biological effects on in vitro and in vivo systems from their exposure to various air suspended and transmitted stimuli (including tobacco smoke and vape aerosols)

Although the InExpose allows generating aerosol by any vape device, their default is (at least up to late 2024) the Evic Mini, a third generation device released in 2015 by JoyeTech. It was among the first ones to allow puffing with power and temperature control modes. The InExpose allows for the selection various coils and alloys with resistances of 0.15, 0.5, 1.0 and 1.5 Ohms.

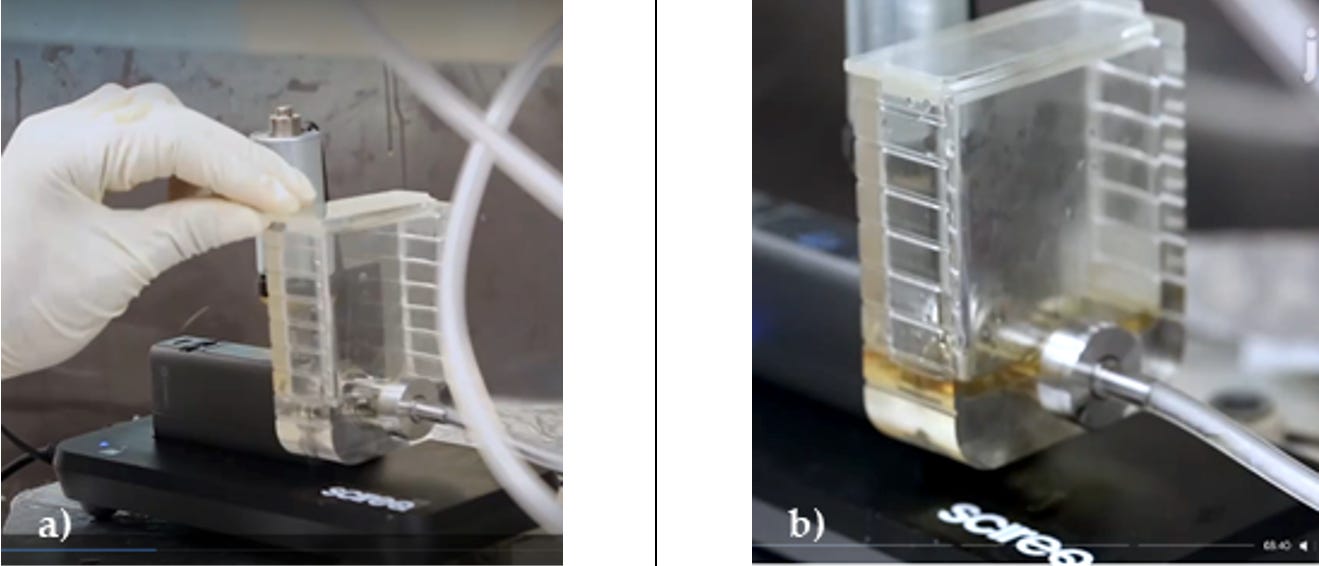
However, the InExpose uses as default only the “mod” (battery plus electronic circuitry) of the Evic Mini, replacing its original atomizer with a custom made 7 mL tank (see video in this published article). The following images were taken from this video
Users of the InExpose may program puff profiles, duration and frequency, but its maximal instantaneous peak flow is 1.675 L/min, leading to airflow rates restricted to around 1-2 L/min for puff durations around 2-4 s.
Calibration tests
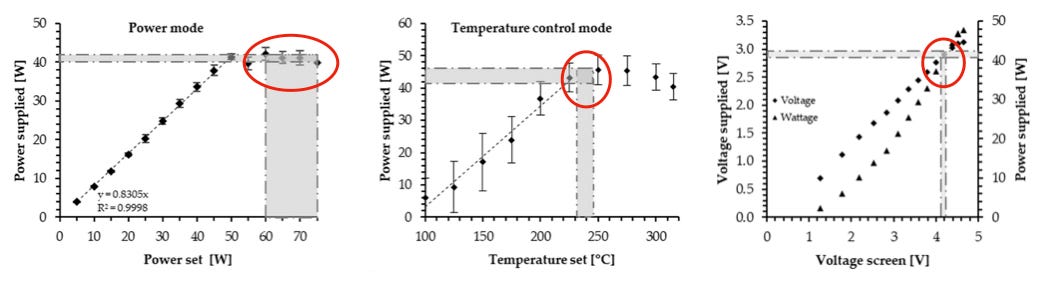
The instrument screen of the Evic Mini displays user selected power and temperature values (in their resective modes). Authors using the InExpose mention these values in their methods section (though authors often fail to mention them). However, It is necessary to validate them in the laboratory (and to validate also the coil resistance). This calibration was undertaken in a previous article by Soulet, Constans and Quinty, that showed considerable discrepancies with repect to the instrument screen values. The results of this calibration for the coil with 0.15 Ohms can be appreciated in the following graphs (reproduced in the review under revision):
The shaded areas in the calibration graphs in figure 4 describe the power, temperature and voltage declared by at least 14 of the authors (from the instrument screen) contrasted (red circles) with the laboratory measured values in the vertical axis. Authors set power at 60-80 W, temperatures at 220-250 °C and voltage at 4-4.8 V. The calibration shows that all these screen instrument values correspond to measured supplied power of 40-45 W. A separate temperature calibration (not displayed) shows measured coil temperature of 300 °C for screen values 220-250 °C. Given this calibration, the next stage is to verify if under these power levels, coil resistance of 0.15 Ohms and the allowed airflow 1-2 L/min the Evic Mini is in the Optimal Regime.
Overheating, aldehyde load and other issues
As mentioned in Post 2, the Optimal Regime is characterized by a linear relation between the mass of e-liquid vaporized (MEV) and supplied power for a given airflow rate. Departure form this regime is also marked by an exponential increase of aldehydes. This test was carried on by Soulet, Constans and Quinty and reproduced in the review under revision. The resulting graphs are:
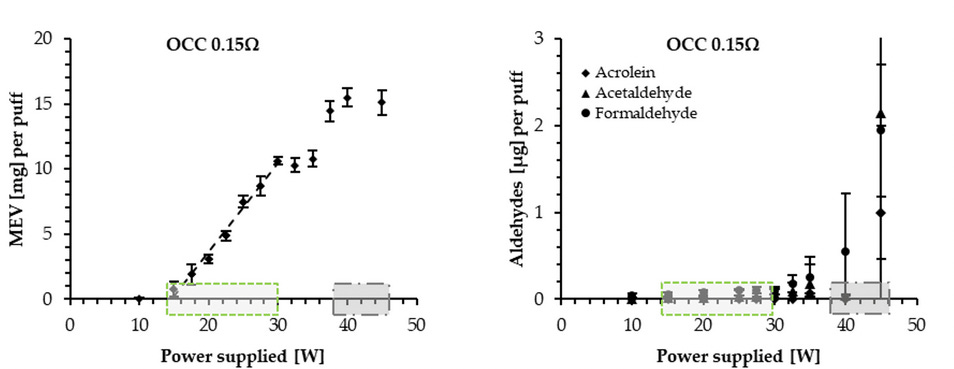
By supplying 40-45 W (dark shaded region) it is evident that the Evic Mini was operated in the Overheating region above the Optimal Regime 14-30 W (light shadowed region). Therefore, we can state with full certainty that at least 14 studies exposed cell cultures and rodents to overheated aerosols loaded with carbonyls. We can also state with full certainty that the reliability of the biological deleterious effects found in the studies is questionable.
A signal of overheating can be appreciated in the published video explaining the operation of the InExpose. As shown in the figure below, at the beginning of the experiment the e-liquid is transparent (panel a)), while it takes a brownish color at the end (panel b)).
This change of color is a signal of the onset of the pyrolysis of the cotton (cellulose) in the wick. This pyrolysis is associated with a “dry puff”, however it is a gradual process that initiates at 300°C with the release of organic compounds that modify the chemical composition of the e-liquid. As we found in the calibration tests the coil temperature reaches 300°C even when the instrument screen displays temperatures below 250°C. The temperature 300°C is above the boiling point of any PG/VG liquid mixture well into the Overheating Regime. Also, the horizontal position of the mod might impair the capilarity of the wick, increasing the risk of a dry puff. CORESTA recommends placing the device at 45°
Besides overheating and aldehyde contents, puffing a sub-ohm device with airflow rates of around 1 L/min is unrepresentative of consumer usage of these devices, which is iteslf currently marginal. This questions the utility of using these devices in any toxicological testing.
Another problem we found in some of studies that we reviewed was puffing the Evic Mini (a high powered device) with e-liquids containing nicotine salts with high concentrations 20 to 36 mg/mL. The authors using these liquids justified it as an effort to mimic smokers with intense nicotine dependencee. This is completely mistaken and is at odds with consumer usage. The delivery of such high nicotine levels to highly dependent users is always through low powered devices (pods and disposables), while conversely high power devices are used with low nicotine levels (nicotine base at < 6 mg/mL). High powered devices generate a large aerosol mass per puff (20-50 mg), thus such high nicotine concentration delivered with the typical “direct to lung” deep inhlation is bound to overload users.
Information vacuum and reproducibility
An esential quality criterion of experimental science is the certainty (or the best possible approach to certainty) to be able to reproduce and/or replicate all experiments. This criterion requires authors to provide all information on experimental procedures for anyone with the appropriate instruments and resources to be able to redo the experiments.
Of the 40 preclinic studies we found in the literature search all biological protocols and associated experimental procedures are well described in full detail. However, only the 14 studies listed in figure 1 provided sufficient information on the experimental procedures of aerosol generation (coil resistance, supplied power or voltage, airflow rate, puff duration and frequency, or coil temperature).
The 14 studies mentioned above are reproducible and replicable (at least 3 of them are). The information on aerosol generation in the remaining 26 studies is too scant to be able to make any inference beyond speculation. These 26 studies are unreproducible and unreplicable. This is a serious flaw in experimental research, though it seems the authors are unaware of this problem, believing there is no problem with the experiments as long as the biological protocols have been well conducted. The lack of considertation of reproducibility by not disclosing aerosol generation parameters seems to be common in preclinic studies. This is a problem that the peer reviewing process must address.
Funding
The overwhelming majority of the 40 studies we found were funded by the US National Institute of Health (NIH), sometimes directly snd some times through the FDA CTP or other institutions (universities) in the USA. Less than 6 studies were partially funded by Chinese, Canadian and German institutions. We estimate a total funding well over 10 million USD. The following table shows the funding sources of the 14 studies listed in the table of figure 1 (all are US NIH and US university funds, save two shared funds from Germany):
Publication blues
We have experienced already 4 rejections of this manuscript in Toxicology journals. The manuscript has been aproved by about half of the reviewers, some even praizing it, but the other half rejected it expressing rude judgemental statements and alluding to our “conflicts of interest”. The editors were abusive and incompetent, hiding information and dismissing favorable reviews , while their final decisions essentially parroted (some times verbatim) the comments of the negative reviewers. This issue deserves a separate Substack that I will release in the near future. Nevertheless, we continue trying.
However, some of the negative reviewers raised an interesting issue: that cells and mice are exposed to a “diluted” (and thus “cleaned”) aerosol that passes through a various tubular conducts connecting the device with the exposure chamber. This is a half truth, the aerosol is evidently diluted in air but this does not eliminate toxic compounds in the gas phase (such as aldehydes and VOCs). Passing through a conduct primarely affects the particulate phase (droplets), with larger ones colliding and settling in the conduct walls. In fact, one of the studies we reviewed (Muthumalage and Rahman) sampled the exposure chamber of the mice and detected high amounts of aldehyde and VOCs. We mentioned this fact in responding to these reviewers, with no avail.
Conclusions
Unfortunately, of the 40 studies that declared having used the InExpose, only 14 provided sufficient information on the parameters of aerosol generation. Information in the remaining ones was from insufficient to none, making them unreproducible.
Using the previously published calibration tests of the Evic Mini we were able to determine that (at least) all these 14 studies exposed cells and rodents to overheated and adehyde loaded aerosols. Regarding the rest of the studies, this conclusion depends on the available information supplied by authors. We found another 67 studies that incurred in the same methodological flaw but did not use the InExpose. We will deal with them in the future.
We regard the InExpose as a very valuable instrument to examine exposure form vape aerosols, but SCIREQ must update its default device (in case it has not done so). We question the utility of using the Evic Mini or other sub-ohm device in preclinic studies, when consumer usage of such devices is currently marginal.
The 14 studies using the InExpose that we were able to reviewed are not useful to assess the safety of vaping, as they have followed a puffing protocol that generated overheated aerosols and that is completely at odds with the consumer usage of sub-ohm devices for the “direct to lung” style that involves large airflows. The same conclusion applies to the 67 studies we found that did not use the InExpose.